
The chances of survival turn slimmer when you are hard to hear each other when speaking. We all know that communication is a vital part of survival, most especially when seeking help or surviving with family members. Old-manufactured gas masks prevent a user from uttering words clearly. If an individual inhales this gas, they can experience shortness of breath and cough from lung irritation.
#Entropy of vaporization skin#
It is a chemical warfare agent that can cause skin burns and severe blisters. Yes, you read it right! This full-face gas mask can protect you from nuclear fallout.Īside from being temporary protection from radioactive particulates, harmful gasses, and vapor, this gas mask can also hold out against mustard gas. Mira Safety CM-6M Gas Mask is ready for Chemical Warfare Agents ( CWA) and CBRN agents.
#Entropy of vaporization how to#
How to Survive During a Nuclear ExplosionĪ Comparison of The Best Nuclear Gas Masks How Do Gas Masks Protect You from Dangerous Environments Things You Need to Know Before Buying A Nuclear Gas Mask The Best Gas Masks for Nuclear Explosion and Fallout Quick NavigationĪ Comparison of The Best Nuclear Gas Mask With those things in mind, we created this article for you to know the top 4 best nuclear gas masks and other relevant information to help you survive when nuclear fallout occurs (hopefully not!). Plus, nukes radiate different types of energy after they have been detonated.
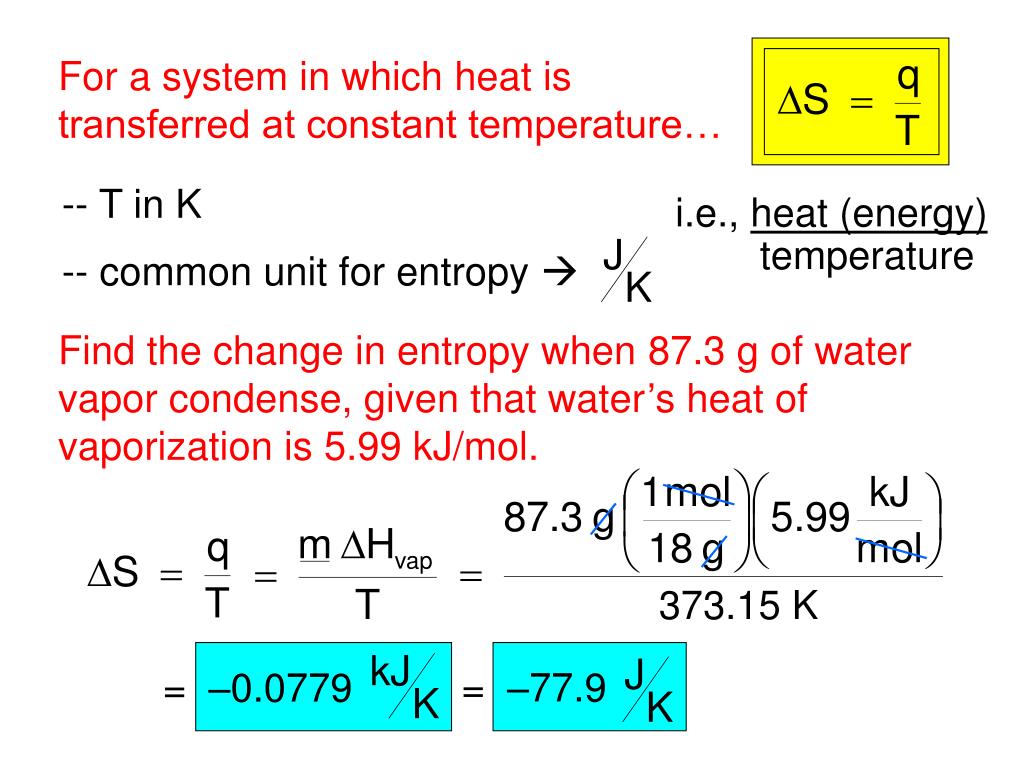
Radiation piggybacks on the ashes and specks of dust carried by the wind may penetrate a person’s body through respiration. The entropy change is negative which is what we expect when going from a liquid to solid state.Surviving a nuclear detonation is one thing, but saving yourself from the harmful effects of the fallout is another. b) What is the entropy of the gaseous chloroform at this temperature if the standard entropy of chloroform is 295.6 J/(mol K)? a) Given that the heat of vaporization of chloroform is 29.24 kJ/mol, calculate the entropy change, S of the system when 1.00 mol of CHCl 3 evaporates at its boiling point (61.2 ☌). The entropy change can be calculated using this equation as long as the temperature remains constant.įor example, Chloroform, CHCl 3, is a common organic solvent once used as an anesthetic. Δ S is the entropy change of the system measured in J/K, and T – the temperature in Kelvin Rearranging the equation, we get an expression for Δ S:
#Entropy of vaporization free#
Remember, also that when the system is at equilibrium the Gibbs free energy is equal to zero: To derive an equation for the entropy change associated with phase transitions, we need to remember that these changes occur when the system is at equilibrium. Remember this pattern, and the corresponding terms for each pair of opposite processes: melting/fusion vs freezing, evaporation/vaporization vs condensation.Ĭalculating the Entropy Change of State Changes These are the points of phase transitions where, for example, the liquid turns into a gas even at the same temperature.

Notice how the entropy is still increasing in the regions where the temperature is not changing.
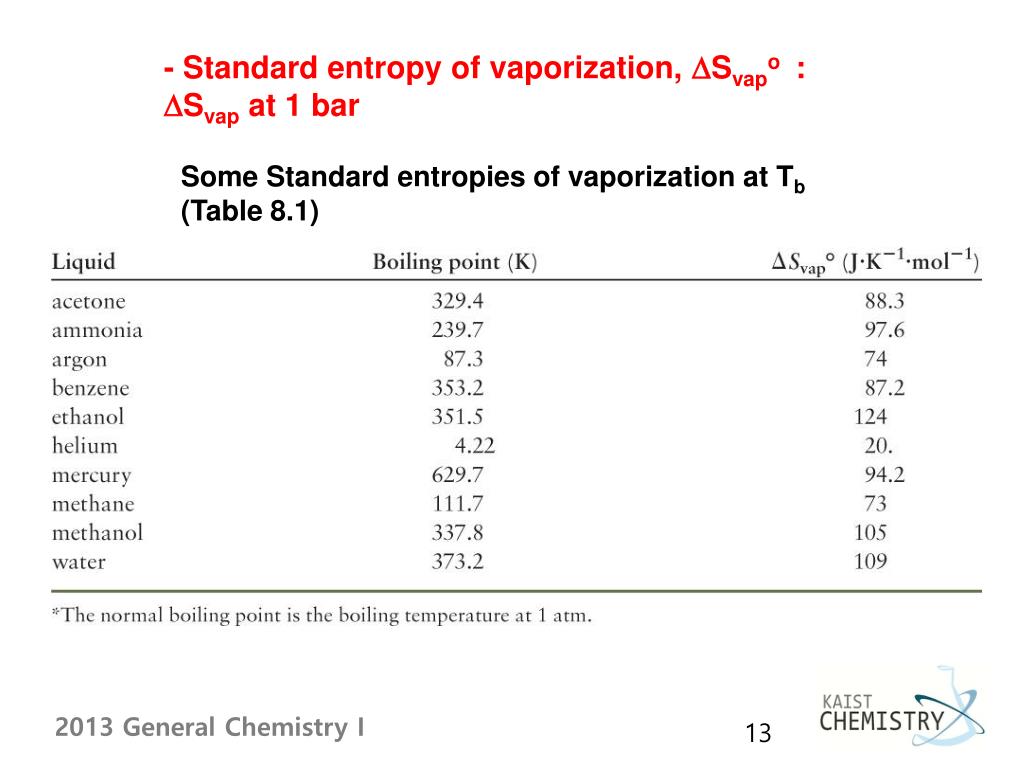
The phase transition graph showing the entropy vs temperature is very useful to visualize this concept: This behavior is explained by the increasing freedom of motion when molecules go from the most ordered solid state to liquid, and then a gas state where the degree of randomness is the highest.

On the basis of determining the entropy change associated with phase transitions is the third law of thermodynamics: the entropy of a perfect crystalline substance is zero at the absolute zero temperature.Īn implication of this is that the entropy is the lowest in solids, and it keeps increasing in the order of going to liquid and gas states: solid < liquid < gas.


 0 kommentar(er)
0 kommentar(er)
